
Candle filter without residual liquid dry discharge
WKZ filter mainly has dryness requirements for filter cake, leaving no residual liquid, and can also be used wet to discharge filter residue for secondary use. Special plum petal filter element design makes filter cake not easy to fall off during filtration, and cake unloading is more thorough.

Classification:
Candle filter

Key words:
Candle filter without residual liquid dry discharge

Product accessories:
Product Details
WKZ Series (No Residual Liquid Dry Row)
WKZ filter mainly has dryness requirements for filter cake, leaving no residual liquid, and can also be used wet to discharge filter residue for secondary use. Special plum petal filter element design makes filter cake not easy to fall off during filtration, and cake unloading is more thorough.
The company uses years of experience accumulation and its own technical advantages to continuously improve the original products. With the help of Professor Su Dazhou Zhufa, the company successfully developed a filter cake thickness detector, which effectively solved the filter cake overload problem.
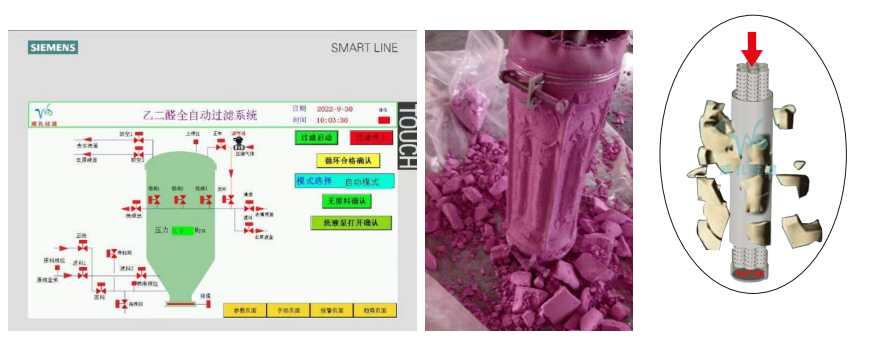
The eighth-generation candle filter provides an intelligent solution using PLC control technology, with six sensors working together.
 1. Completely closed high security system, no liquid and odor leakage
1. Completely closed high security system, no liquid and odor leakage
2. High interception efficiency of solid particles
3. Can effectively intercept sub-micron particles
4. All filter cakes are stably attached to the filter cloth. Slurry or dry slag discharge
5. The residual liquid of the filter can be atomized and sprayed to dry pressure. Achieve no residual liquid
6. To slag purification, improve product quality
7. Precious metals/catalysts. Filtrate residue co-recovery
8. Can be online circulation spray washing filter cake
9. More than one hundred kinds of filter cloth can choose
10. Filter cloth back-blowing regeneration effect as before
11. Stable operation at minus 30 ℃ to high temperature 260 ℃
12. If the process needs to add filter aid, the space of the filter is fully satisfied
13. Pharmaceutical, biochemical, food industry can also meet GMP requirements, steam or chemical method SIP sterilization.
More than 1000 filters are in operation across the country.
Previous Page
Next Page
Previous Page
Next Page
